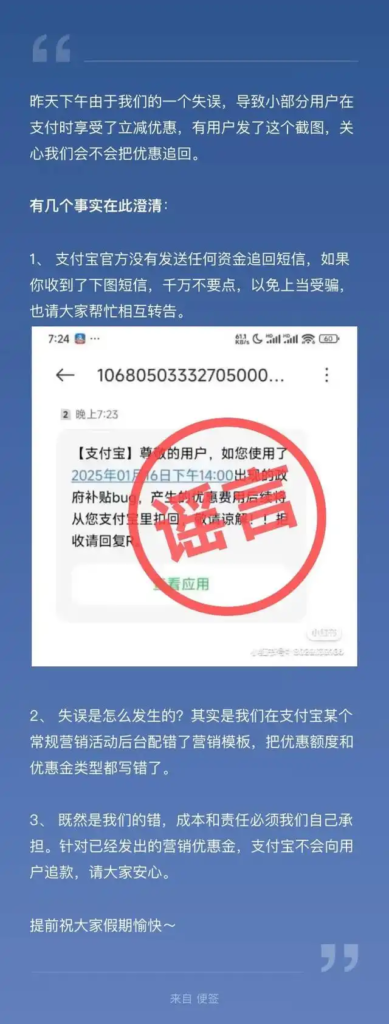
BEIJING, February 26 According to the website of the National Medical Products Administration, recently, the National Medical Products Administration has approved the registration application for the innovative products of the Heart Pulsed Electromagnetic Field Ablation Device and the One-time Use Heart Pulsed Electromagnetic Field Ablation Catheter of Shanghai Xuanyu Medical Devices Co., Ltd.
The cardiac pulsed radiofrequency ablation device consists of a main unit and accessories. The accessories include AC power cord, foot switch, electrocardiogram lead wire, and equipotential line. The disposable cardiac pulsed radiofrequency ablation catheter is composed of electrode, electrode tube, catheter body, handle, connector and connection cable.
The two products are used in combination. By generating an electric field through high-voltage pulses, atrial fibrillation can be ablated, causing irreversible electrical perforation and necrosis or apoptosis of the diseased cells. This product can selectively damage the myocardial tissue, avoiding the risk of damage to surrounding tissues caused by heat transfer, and benefiting more patients with drug-resistant, recurrent, symptomatic, and paroxysmal atrial fibrillation.
The drug supervision and administration department will strengthen the post-marketing supervision of the aforementioned products to safeguard the safety of patients’ use of medical devices.

 Entering China
Entering China