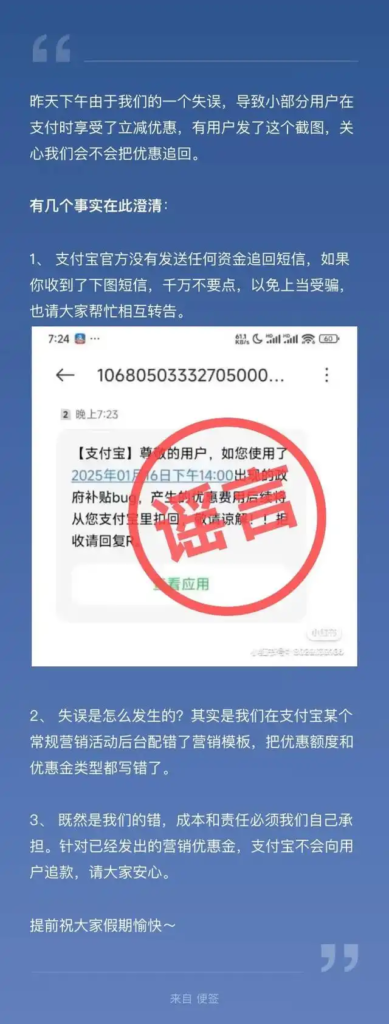
Recently, during the “Two Sessions” in Shanghai, some CPPCC members and medical experts reported that some centralized procurement drugs may have quality risks and other problems, and the National Health Insurance Bureau attaches great importance to them. The National Health Insurance Administration today (20th) announced the “Letter on Assisting in Listening to Relevant Opinions on Centralized Procurement of Drugs” to the Shanghai Municipal Health Insurance Bureau.
The letter stated that in order to effectively accept democratic supervision, listen to the voices of the clinical frontline, give full play to the professional role of doctors, obtain first-hand direct evidence of the clinical effectiveness of centralized procurement of drugs, further maintain the order of centralized drug procurement, and safeguard the health rights and interests of the people, it was decided that on January 21, the responsible comrades of the National Health Insurance Bureau led a team to go to Shanghai to listen to relevant members in person in conjunction with the health, industrial informatization, and drug regulatory departments. The opinions and suggestions of experts on the centralized procurement policy of drugs and the quality assurance of the selected products, and focus on collecting clues on the quality and efficacy of drugs supported by clinical data and with statistical differences.
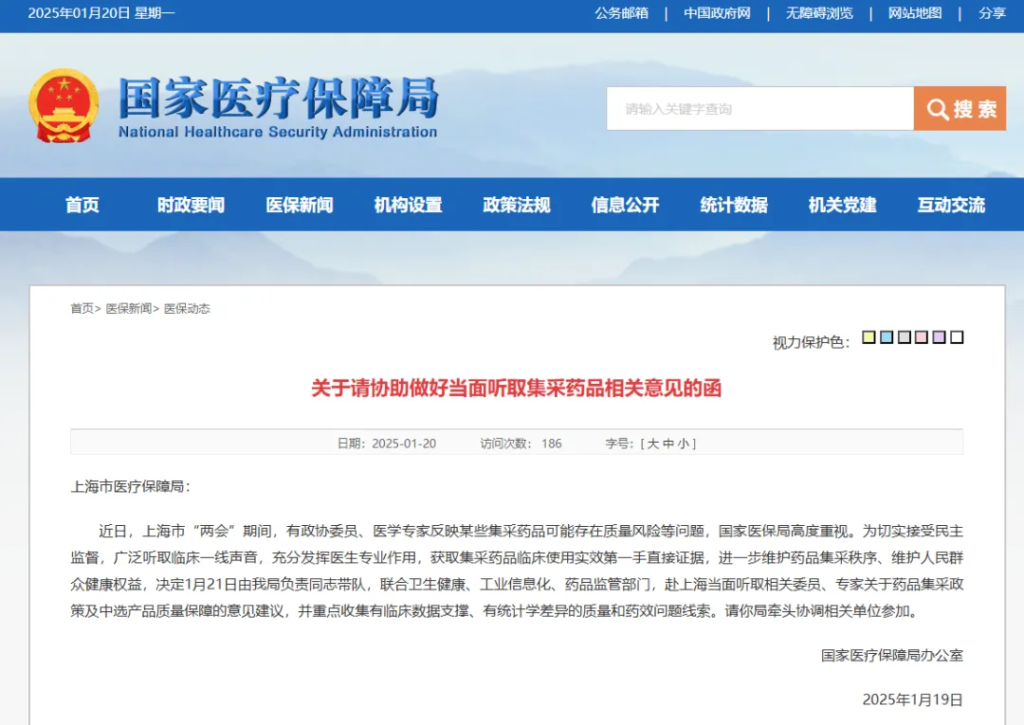
The letter also published an outline of exchanges with relevant people in Shanghai.
1. Introduce the basic policy and quality assurance of centralized drug procurement. Reliable quality is a prerequisite for the inclusion of drugs in centralized procurement; Reference preparations (mainly original drugs), as well as generic drugs that have passed the quality and efficacy consistency evaluation organized by the State Food and Drug Administration, can be included in the scope of centralized procurement. Centralized procurement by the national medical institutions to fill in the number of purchases, enterprises independent quotation competition, after the selection of the signing of the purchase agreement, the medical insurance department to urge the selected enterprises to ensure the supply according to the agreement. The amount of centralized procurement agreement is generally 60%-80% of the volume reported by medical institutions, and the remaining part is independently selected by medical institutions to purchase brands, and there is no “one-size-fits-all” institutional arrangement that does not allow the procurement and use of “imported original drugs”. Introducing the consistency evaluation of generic drug quality and efficacy and the participation of hospitals in research and trials, the centralized procurement bidding enterprises have passed the consistency evaluation and accepted the 100% full coverage inspection and sampling of enterprises and varieties by the drug regulatory department. This paper introduces the real-world research of selected drugs carried out by more than 80 tertiary hospitals covering more than 300,000 patients. Introduce the disposal of individual selected drugs that have found quality problems.
2. In-depth verification of the clinical effectiveness of centralized procurement drugs. Listen to the clinical frontline’s feelings about the effectiveness of the use of the selected drugs in centralized procurement, and focus on collecting cases where there are differences between the selected generic drugs and the original drugs in terms of efficacy and safety indicators such as cure rate, treatment effective rate, and incidence of adverse reactions, and there are statistical significance and case information can be traced. According to the generic name, dosage form, specification, name of the selected enterprise, effectiveness and safety information (such as antihypertensive drug systolic blood pressure, diastolic blood pressure value, anti-tumor drug progression-free survival, recurrence rate and metastasis rate, hypoglycemic drug glycosylated hemoglobin compliance rate, fasting blood glucose compliance rate, liver and kidney function indicators, etc.), the list of problem clues is summarized and formed, and it is officially handed over to the drug quality supervision and management department. At the same time, understand the specific situation that the experts reported that the supply of some of the selected drugs was not timely, and resolutely urge the selected enterprises to correct them, and those that cannot be corrected in time will be disposed of according to the bidding agreement.
3. Discuss opinions and suggestions on further ensuring the quality and effectiveness of centralized drug procurement. If there are indeed quality problems in centralized procurement of drugs, the medical insurance department will resolutely investigate the responsibility of the selected enterprises in accordance with the centralized procurement agreement and relevant systems, including but not limited to canceling the qualifications of the winning enterprises, being included in the list of centralized procurement violations, and giving dishonesty ratings. At the same time, they discussed and explored effective ways to further ensure the quality and efficacy of centralized drug procurement, including the three suggestions in the proposal of the Shanghai Municipal Committee of the Revolutionary Committee of the Chinese Kuomintang reported by the media, such as requesting the competent department of drug quality supervision to strengthen the daily inspection of drugs after passing the consistency evaluation, completely publicizing the bioequivalence test results of the consistency evaluation of generic drugs, and establishing feedback collection channels for comparative evidence of drug efficacy in medical institutions.

 Entering China
Entering China